The collaborative nature of public health was on display at the WSLH Newborn Screening Laboratory in October as Dr. Ellen Stevens from the North Carolina Public Health Laboratory (NCPHL) spent a week learning the next-generation sequencing assay the WSLH performs for cystic fibrosis (CF) screening.
Last year the vendor that produced the type of assay the North Carolina lab was using for CF screening testing decided to remove it from the market due to quality issues. NCPHL needed help quickly to continue CF screening and the WSLH stepped up since we use the next-generation sequencing method and weren’t affected. Dr. Stevens’ Wisconsin visit was to learn this testing method and reporting process to help NCPHL establish it in-house.
According to WSLH Newborn Screening Laboratory Co-Director Dr. Mei Baker, the Wisconsin and North Carolina newborn screening programs have worked together for many years.
“Our helping them this past year is just a continuation of our long-time collaboration,” Dr. Baker said.
“Mei and the WSLH staff have been amazing at helping us get going on next-gen sequencing for CF,” Dr. Stevens notes. “Public health is all about helping each other to help people.”
Dr. Stevens is the current APHL-Ronald H. Laessig Newborn Screening Fellow.
Dr. Laessig was WSLH Director from 1980–2006 and a national leader in newborn screening. After his death in 2009, the Association of Public Health Laboratories (APHL) created the fellowship in his honor.
WSLH Newborn Screening Laboratory Co-Director Dr. Patrice Held was the first Laessig fellow.
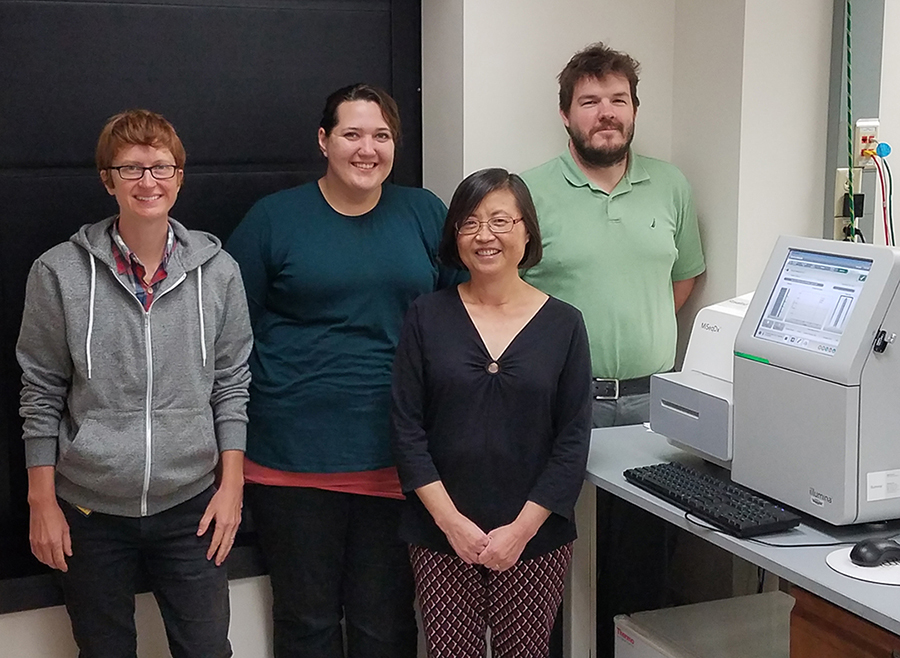
From left: Dr. Ellen Stevens from the North Carolina Public Health Laboratory, WSLH Chemist Bethany Zeitler, WSLH Newborn Screening Lab Co-Director Dr. Mei Baker and WSLH Chemist Sean Mochal stand next to the Illumina MiSeqDx, which is used to perform next-generation sequencing screening testing for cystic fibrosis.
